
Miramodus
Molecular models, crystal structure models and mineral structures
Molecular Models. We design, build and sell beautiful premium quality ball and stick molecular models and crystal structure models for teaching, museum displays, presentations, awards, legal cases - or for anyone who just wants a stunning scientific model.

|

|
Models are a fascinating and engaging part of chemistry teaching, and are a useful tool for explaining chemical concepts or material properties to colleagues and customers. They provide a tangible interactive means for educators to demonstrate the relation between molecular structure and chemical function in a form that cannot be replicated with, for example, computer imaging. Similarly, they usefully illustrate the relation between mineral structures and physical or chemical properties. Alternatively, they can simply be works of art - three dimensional sculptures that enrich a work or visitor area. Whatever you need them for, our models are accurate, look stunning, and will enhance any office or laboratory.

Examples of our work can be seen throughout our website. Please use the links above to find out more about our products, search our catalogue, or to inquire about having us make a model of your structure.
We hope you enjoy looking round our website and seeing the quality and variety of stunning scientific models that we make for display and education. To buy models from us, or to discuss what you would like made, please either complete an order form or the contact form, or email us at [email protected].
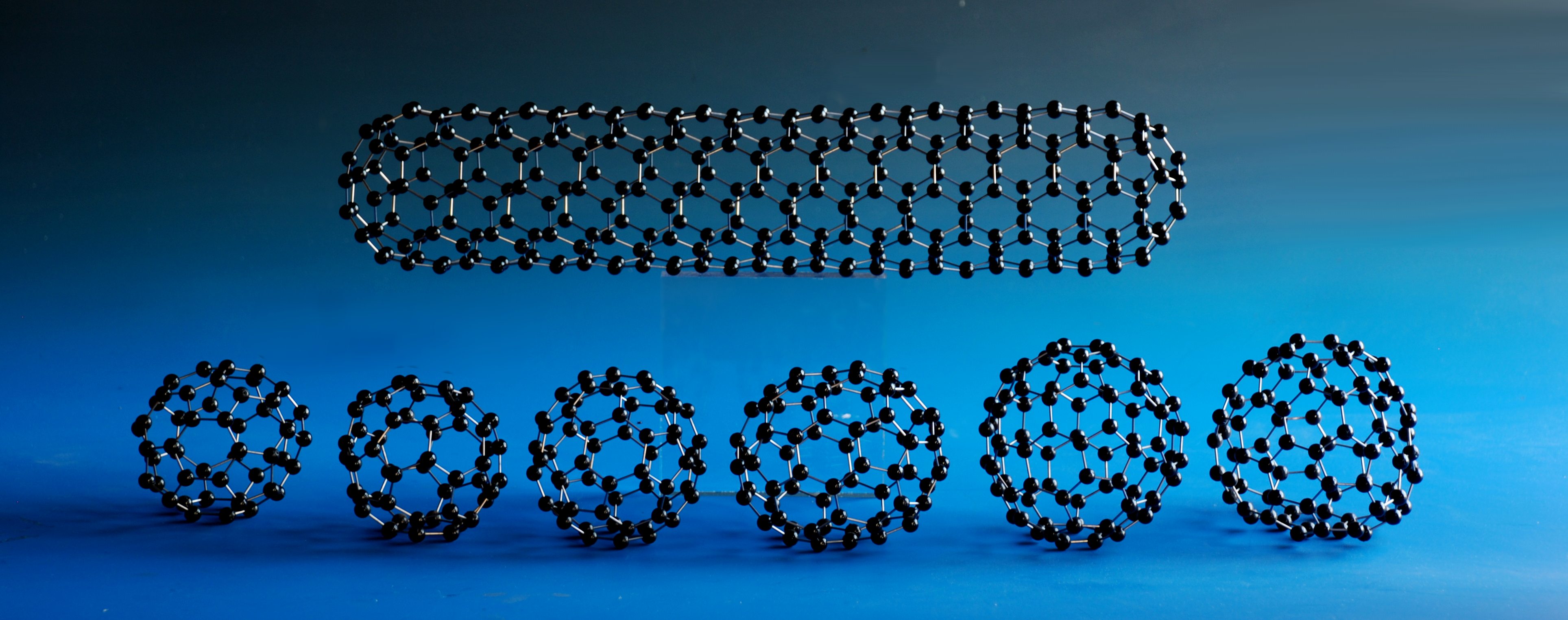
Pre-constructed Models
Pre-constructed models represent the vast majority of our sales, and they are some of the finest crystal structure models that can be purchased. We have the widest range of scales of crystal structure models in the world, ranging from a scale of 1cm=1Å (“Beevers’ Models”™) through to our giant molecular models at a scale of 20cm=1Å or greater, and in a huge range of materials. Almost all of our models are made to order, with the exception of a small number of the commonest chemical structures that we keep in stock, and nothing is 'standard' about them. If a bond angle in a "tetrahedrally" coordinated atom in the structure is 112°, the balls are drilled with holes arranged at that angle - we don't insist that you have to compromise by using regular tetrahedral coordination. The fact that we work with that accuracy can make all the difference between representing features that exist in a crystal and missing them out entirely. What's more, because our models are all made to scale, you also get to see the relative sizes of crystal structures - with regular model kits, for example, a diamond unit cell is much larger than that of sodium chloride, whereas in reality (and in our models) the opposite is true.
Beevers Models (named after their creator, Arnold Beevers) are made with acrylic balls and steel rods, but we also work with a wide range of materials to suit your purpose and your budget. We have made and sold molecular and crystal structure models in acrylic, phenolic resin, wood, brass, copper, aluminium, silver and a host of other materials.

A model of Gallium Nitride made with brass and aluminium balls, linked by green anodised aluminium rods on a granite base.
The accuracy and compactness of our Beevers Models™ system allows our model range to extend over the entire spectrum of 3-dimensional structures in Chemistry, Biology, Geology, Physics and Mathematics. Our huge list of Beevers' ball and stick models for sale from our catalogue includes crystal structure models, biological, physical, mathematical and crystallographic teaching models. We also make an extensive range of models of organic and inorganic molecules - or any compound you want to order. All the usual favourites are available - you can buy a molecular model of DNA or of RNA, inorganic molecules such as fullerenes (buckyballs), from C28 through C60 and C70 to C720 and beyond, inorganic structures such as NaCl or YBCO, and innumerable materials you have probably never encountered, such as Thorium Zinc Carbide. Even if the material that you want is not in our catalogue - or you want a molecular model of an entirely new compound - we will still be able to make your structure, whatever its size; all we need are the crystallographic details or node coordinates.
Multiple bonds in our models
All of our larger scale models (including our giant molecular models) can be made with double bonds, triple bonds or quadruple bonds as part of the structure. We have never attempted quintuple or sextuple bonds and, given their extreme rarity, we don't anticipate ever needing to, but we could include them in larger models if it were ever necessary. Similarly, we can include multiple bonds in allenes and similar structures. How the rods are arranged in triple and quadruple bonds is generally at the customer's discretion, but our default design is to have the bonds symmetrically arranged around the bond axis, rather than to have the rods in a line. So, a triple bond cut across its axis would have three rods at the corners of an equilateral triangle rather than looking like a double bond with an extra bond added in the middle. A quadruple bond would be arranged with either four bonds in square, or a central bond and three surrounding bonds in an equilateral triangle.
Because of size constraints, we do not currently make Beevers models with multiple bonds, but we are hoping to be able to introduce these with new equipment later in 2020 - where necessary, we currently indicate double bonds in Beevers models by using thicker (2mm diameter) rods, in either aluminium, copper or brass, depending on the customer's preference.
Scale matters!
All our models are made accurately and to scale, making it possible to directly measure angles and lengths, to visualise the relative scale of different structures and to show how, for example, a solvent molecule can enter into a porous structure. With other standard model systems, this simply isn't a reliable option, as the user has no assurance that the solvent and the matrix have been made on the same scale. With our models you can immediately see, for example, why diamond is more dense than graphite and why zeolites absorb some compounds but not others.
Kits
Model kits for any of our models can be supplied on request. Because it is largely impractical to send our large models and giant models out as fully constructed molecules or crystals, we routinely supply these models as kits, albeit kits made specifically for the construction of a particular molecule or crystal. If you want a more general kit of large or giant atoms and bonds, we are more than happy to make those up for you, and you can buy exactly what you want as we sell the atoms and bonds individually. Giant kits are fantastic for teaching, for interactive museum exhibits, and for fun activities.
That being said, we don't generally advise people to buy Beevers models in kit or self-build form. Because Beevers models are largely designed for display or teaching specific crystallographic or structural concepts, they are not designed to be taken apart and rebuilt, and they are not as easy to construct as normal model kits. By their nature, our Beevers models don’t readily lend themselves to being sold as kits but we are prepared to sell them for self-build. The buyer needs to be aware, and accept, that they are not as easy assemble as most people expect.
We specialise in building and supplying ready-made ball and stick models, which represent just one possible type of model for representing molecules and crystal structures. Models are invaluable in allowing researchers to easily illustrate how and why their structure functions in the way that it does, and to better explain their research to visitors. It seems like a ridiculous question if you are used to using molecular models, but have you really thought about what molecular models are?
When I need to explain something, I find it helps to re-examine its dictionary definition. In our case, the Oxford English Dictionary defines a model as:
“a representation in three dimensions of some projected or existing structure, or of some material object, artificial or natural, showing the proportions and arrangement of its component parts”.
Models are common in our everyday lives. Anyone who enters a museum is likely to be surrounded by dozens of them, new public buildings often have an architectural model in their foyer, and many people still build the "Airfix" or "Revell" style of transport model. These models are almost always scaled-down versions of larger structures, and are structurally and aesthetically very similar to the objects that they represent – they're just smaller, having been scaled down by factors of between 10 and 1000.
Models of molecules are not like these, though, and it is all too easy to think that they are. The first difference is only obvious when we think about it - we are not scaling down, we are scaling up - and by huge factors: typically by the order of 1010.
The direction and magnitude of the scaling, while curious, isn't a significant difference. The important distinction is that we're not creating enlarged facsimiles of the subject matter, because what we are modelling doesn't exist in the same form as the macroscopic world. We are attempting to produce models of concepts that are the result of mathematical descriptions (take a moment to get your head around that). Those mathematical models have assumptions and approximations imposed on them, such as larger atoms having hydrogen-like orbitals. We are creating physical models of a scientific model of a mathematical approximation. These aren’t models in the engineering sense; these are now illustrations of descriptions of reality.
Molecular or crystal structure models, then, can never be perfect replicas of the microscopic structures that they represent. Unlike engineering models, the qualitative difference between the quantum world and the macroscopic world is an unbridgeable gap because we cannot create a scaled replica of that particular reality - and if we could, it wouldn't really help much anyway.
What do we want from a molecular model?
When we construct a model of a crystal structure or a molecule, therefore, we must first decide on the purpose of the model. Any worthwhile model has a purpose - and if the purpose of a molecular model cannot be that of a facsimile of the real thing, then it must lie in the representation of specific aspects of the molecule.
There are a number of different types of molecular model, and each can be used to illustrate different aspects of the molecules that they represent, but there are two fundamental things that they show: either the position of the nuclei and the connections between them, or the volume occupied by the electrons. The former are represented by ball and rod structures or framework models, and allow visualisation of the atom positions in relation to one another and whether or not there are bonds between them. Space-filling models, on the other hand, allow visualisation of the volume occupied by the molecules (but bear in mind that molecules comprising diffuse electron clouds don’t have hard boundaries). There are other specialist forms, of course, such as polyhedral structure models, that are used to illustrate the coordination polyhedra around cations.
School is where most of us start learning about molecular structures. We are taught that we can draw structures comprising element symbols that we join together with lines and, later, that we can combine some groups in units, such as -CH3. At this stage of a science student’s education, space-filling models usefully show how the simple 'structures’ written on paper representation three-dimensional electron clouds that are held in check by quantum properties (although we just tell school students that they have these shapes and they should accept that for now…). As the nature of electron orbitals and chemical bonds become more familiar to us, the size and shape of the electron clouds in molecules (and hence the shapes of the molecules themselves) become implicit in our minds as we scribble down a chemical structure. It's unlikely that many of us give a second thought to this process, but just think for a minute - when you draw a trivalent nitrogen structure, don't you just know there's a non-bonding electron pair in there, too? Most of the time, then, it's enough to represent the relative positions of the nuclei – just as a paleozoologist can look at a skeleton of an extinct animal and visualise the muscles of the living creature, how it held itself and how it moved, chemists automatically ‘fill in’ the electron clouds and corresponding bonds for themselves. This is incredibly useful – that we can, to a significant extent (and without having to spend much conscious thought), extrapolate the three-dimensional electron clouds in a molecule from the positions of the nuclei, enable us to learn more from these ‘skeletal’ structure models than might appear to be the case.
This explains why ‘ball and stick’ (or ‘ball and spoke’) models can be so useful in our teaching, and why they remain the most common form of molecular model. Chemical function is affected by shape and form just as much as it is by aspects such as charge separation - and because you can see right through a ball and rod structure, and you can see 'atoms' on the far side of the model means that the model’s teaching potential is enhanced. The user is able to clearly see the nature and type of atoms that make up the structure through the whole structre, along with the the manner in which the atoms are joined together to form the shapes that those linkages produce. If we represent the structure with space-filling models, we would normally be unable to see beyond the outermost layer of atoms. Being able to look through the structure and into its interior allows huge amounts of structural information to be conveyed in a simple, elegant and efficient form.
Our models
This is where companies like ours come in. In previous times, many chemists made their own models, but that was in an age when research worked more slowly, academic pressures were lower and people had more time to spend making the models. Our role is to make those models for businesses, museums, academics, individuals, lawyers, artists - anyone who wants a fine-looking model.
Ours is a business that was started by Arnold Beevers, an early crystallographer, who started the business as a unit around forty years ago within the University of Edinburgh. After Arnold's death and some uncertainty about its future, I had the opportunity to take over 'Beevers Models’ - the alternative was that it would have simply ceased to exist. That would have been a tragedy - Beevers models are found in museums, universities and businesses around the world. As the late Victor Kiam, former owner of Remington put it; "I liked [the product] so much, I bought the company". We employed new staff, and continued for while in the University of Edinburgh’s School of Chemistry, where I lectured for several years. A few years ago, we moved out and are now based in the Scottish Borders, completely separate from the university.
Our business focuses on ball and spoke models of inorganic structures and small organic molecules. We now build giant models, metal models, Perspex (Lucite) models, 3d printed models, medium scale model kits, and any new challenges that customers like to throw at us.
The end of molecular models?
It's a common question - how long do we think that model-making will last in a world of increasingly realistic computer graphics? There is an issue with computer graphics, though, because they don't perfectly represent 3d objects to our eyes - our vision results from a far richer experience than two slightly dissimilar images viewed in each eye. Our perceived depth of field and the need to be able to instantly refocus enhance our experience. The three dimensional nature of physical models is more easily viewed than even the best computer graphics.
It is a wonderful irony that we sell a good number of models to theoreticians who can’t easily visualise the interactions in, or geometry of, the molecules that they are studying, or can't easily show them to visitors. We get many, many, emails from customers thanking us for their models - most often for their beauty but often for new insights that they have given them.
Ultimately, many people simply take pleasure from owning tangible and elegant structures that illustrate their research interests. It’s part of being human - people have made and valued decorative objects for tens of thousands of years and that's unlikely to change soon. For myself, I've enjoyed and appreciated molecular models for as long as I can remember - it’s why I build them, it's why I run this company, and it's why we do our best to produce the best possible models for others to enjoy. As long as people want to have their crystal structures, and molecules in the form of sculptures that they can display, we’ll be here to make those models for them.
Face Centred Cubic structure model of Copper
Company Registered in Scotland no. SC329683 VAT no 916067916